SPARK scholars pivot to COVID-19 research
Posted on January 29th, 2021
In the last year, as labs were closed and research was halted on ongoing projects, many researchers turned their attention to fighting the COVID-19 pandemic using their own skills and knowledge. This includes the many scientists who have had or now have a project supported by the SPARK at Stanford translational research program.
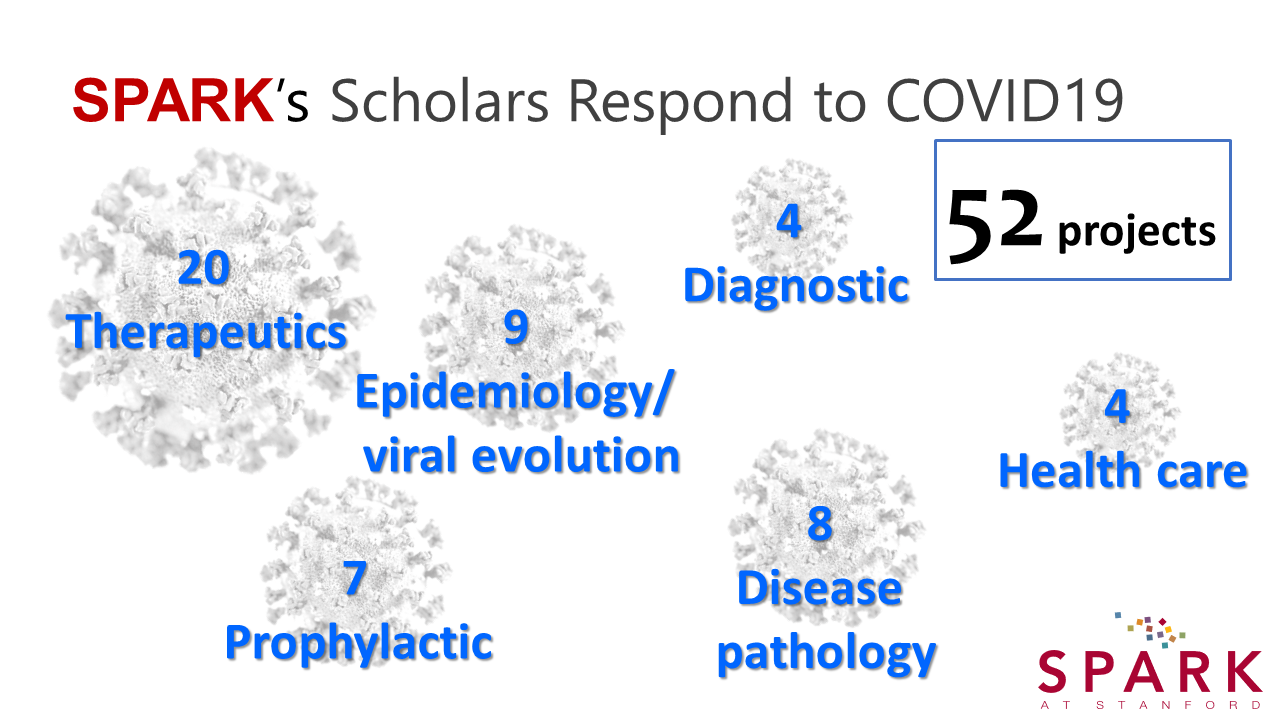
We were aware that current and past SPARK scholars had pivoted their research to address COVID-19 in the last year, but did not know the scope and details of these studies. Early this month, we emailed a request for information to over 300 current and former SPARK scholars asking them to provide a brief description of their COVID-related work over the past year. We received responses describing 52 projects in the following categories: therapeutic development; prophylactic development; diagnostics; study of viral transmission and epidemiology; study of disease and pathology; and effects on healthcare.
Some respondents noted that the tools acquired through SPARK facilitated their work addressing this urgent need. Many also said that their studies have been negatively impacted by lack of funding.
Some highlights of the projects follow.
Among the 20 therapeutic development projects, SPARK co-director Kevin Grimes is repurposing ulinastatin and angiotensin-1-7 to treat hospitalized patients with COVID-19. Ulinastatin, a serine protease inhibitor approved for sepsis and pancreatitis in Asia, and the protein angiotensin(1-7) could reduce COVID-19 disease severity by restoring the imbalance in the renin-angiotensin system caused by SARS-CoV-2 infection. Dr. Grimes and his team have worked to get the compounds ready for clinical trials; both have open INDs and are poised to begin clinical testing.
Other projects are also examining repurposed drugs for COVID-19. David Stevenson and his laboratory are performing computationally supported drug repurposing studies investigating the use of the selective serotonin reuptake inhibitors (SSRIs) fluoxetine and fluvoxamine and COVID severity. Dr. Stevenson’s team has done some population-based studies on the use of statins and COVID severity, and are also investigating the role of heme oxygenase gene expression and COVID severity.
Additionally, Shirit Einav is repurposing ErbB inhibitors to reduce SARS-CoV-2 infection, inflammation and tissue injury. ErbB4 is required for SARS-CoV-2 entry, and is a novel antiviral target. Published data indicate that ErbB1/2 are key regulators of acute lung injury/ acute respiratory distress syndrome (ARDS) and fibrosis, and that their inhibition protects mice from lung injury and mortality. Two pan-ErbB inhibitors emerged as SARS-CoV-2 inhibitors in a high-throughput screen, and Dr. Einav’s team is generating datasets to support their FDA IND application.
Of the seven prophylactic projects, one effort is the chicken antibody-based nasal drops that SPARK founder and co-director Daria Mochly-Rosen is developing with the help of SPARK advisors and an international team. The nasal drops, which serve as a passive vaccine for immediate, temporary COVID-19 protection, have been tested in a Phase I clinical trial. The nasal drop prophylaxis comprises egg yolk-derived IgY antibodies against the spike protein to capture and neutralize the SARS-CoV-2 virus at the nasal mucosa. The team is awaiting response from the FDA and further funding to enable executing efficacy studies in humans, to benefit low- and middle-income countries and help stop the pandemic.
Four projects are investigating alternative diagnostic applications for COVID-19 with the hope of developing rapid, inexpensive, more sensitive and/or easily deployable diagnostics. Juan Santiago’s laboratory developed a rapid, 35 minute CRISPR-based assay using electric-field driven microfluidics. The Niaz Banaei lab developed an interferon-gamma release assay for detection of T cell response (as opposed to antibody response) to SARS-CoV-2, and showed it was more sensitive than serology in household contacts. And Matthew Bogyo’s group developed a protease-activated chemiluminescent reporter for diagnosis of SARS-CoV-2 infection. This would allow rapid testing in saliva for SARS-CoV-2 infection using simple and cheap cell phone-based detectors in low-resource areas.
Nine projects are studying viral transmission and epidemiology. With members of her lab and two first year students, Dr. Mochly-Rosen computationally analyzed the >4000 mutations that had naturally accumulated in the spike protein between February and November 2020, to identify regions in the spike protein that are the least mutated and therefore should be targeted for prophylaxis or therapeutics. Dr. Mochly-Rosen’s team also examined the features of two new, more infective, variants (501Y.V1 and 501Y.V2), the so-called U.K. and South African variants, to understand why they may be more infective but not do not increase the severity of COVID-19. Preprints on both studies were published in bioRxiv earlier this month; both are accepted for publication.
Ami Bhatt, working in collaboration with many post-docs, graduate students, and researchers across the Stanford campus, is characterizing SARS-COV-2 viral RNA shedding in stool from patients with mild to moderate COVID-19 enrolled in two Phase II clinical trials at Stanford. Dr. Bhatt’s team aims to quantify fecal viral shedding dynamics over the course of disease in order to inform public health and wastewater-based epidemiology efforts.
Eight projects are studying COVID-19 disease and pathology. Dr. Grimes and his team are studying changes in cardiovascular biomarker levels, including not just ACE2 but also angiotensin II and angiotensin(1-7), in patients with SARS-CoV-2 as biomarkers of COVID-19 disease. Ang II and Ang(1-7) are important signaling molecules in the renin-angiotensin system that is implicated in SARS-CoV-2 infection and response.
Finally, four projects look at changes in healthcare due to COVID-19, and expanded access to healthcare therapies, such as an autism therapy from the Dennis Wall lab and a virtual reality intervention for problematic eating from the Debra Safer lab.
Permalink: https://sparkmed.stanford.edu/spark-scholars-pivot-to-covid-19-research/
