SPARK’s Commercial Successes
Bridging the gap between bench and bedside is a challenging endeavor. There is an inherent risk that early-stage programs will fail during development, no matter how promising the science.
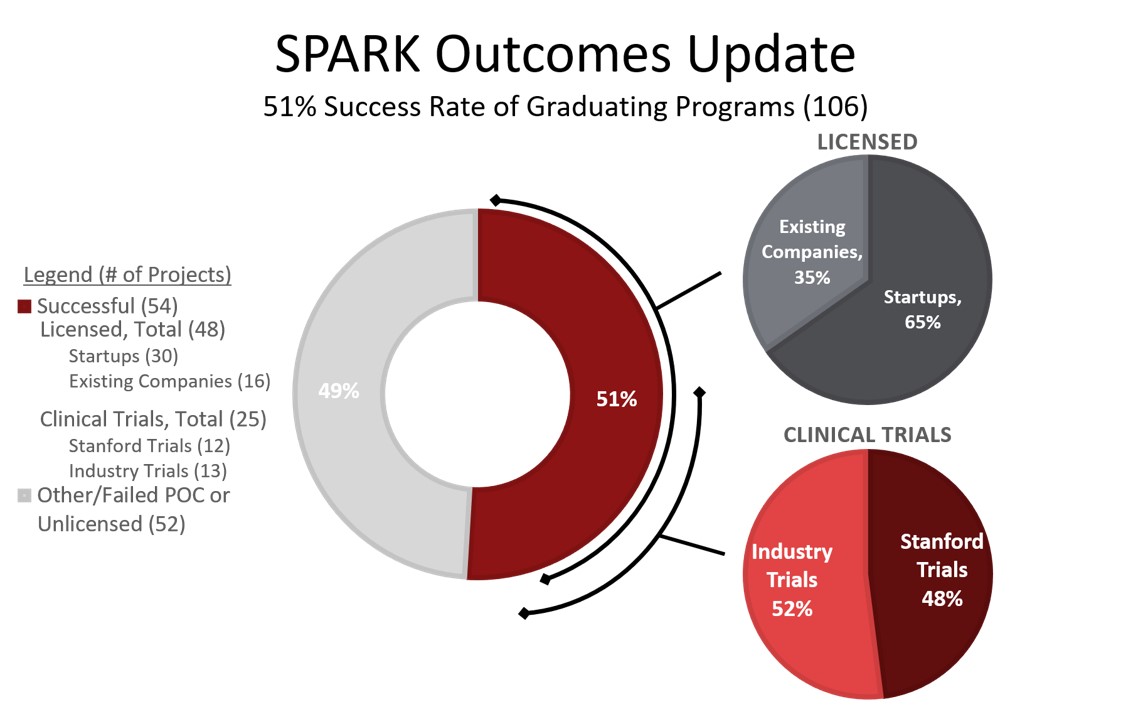
Such nascent programs are unlikely to attract interest from industry until they have reached significant milestones, and very little funding is available from the NIH, foundations, or private enterprise for this critical transition. Nevertheless, SPARK has been able to successfully translate 51% of projects to the clinical and commercial sectors.
Below are some of the commercial success stories:
REPURPOSED AND NOVEL SMALL MOLECULES FOR USE AS ANTIVIRAL THERAPEUTICS
 Shirit Einav, MD
Shirit Einav, MD
Assistant Professor Of Medicine (Infectious Diseases) And Of Microbiology And Immunology
Dr. Einav’s team has identified two human host proteins that are essential for viral life cycle. With SPARK support, the team discovered that a combination of two FDA-approved anti-cancer drugs were effective against hepatitis C, Dengue, West Nile, and Ebola viral infections. During the West African ebola outbreak several years ago, the drug combination therapy was selected along with Z-MAPP and convalescent serum for a clinical study in Sierra Leone that was sponsored by the WHO and Gates Foundation.
Globavir Biosciences, a company committed to delivering antiviral therapies to the developing world, has licensed this two-drug combination therapy and is planning international clinical trials in patients with dengue virus infections. In addition to this drug combination, Dr. Einav’s team identified novel lead compounds with broad-spectrum antiviral activity.
DEVELOPMENT OF SMALL MOLECULE TO TREAT AMYLOIDOSIS
 Isabella Graef, PhD
Isabella Graef, PhD
Asst. Professor Of Pathology
Transthyretin (TTR) is a protein made in the liver and secreted in the blood. There are over 100 known mutations in TTR that bias the protein to toxic amyloid deposits, resulting in peripheral neuropathy and heart failure. Even the wild-type protein is shown to accumulate in aggregates in old age. Dr. Isabella Graef developed a screen to search for small molecules capable of stabilizing the TTR protein in its native tetramer and preventing aggregation of unstable monomers. Her lab was able to identify a novel small molecule that stabilized both the mutant and wild-type forms of TTR. Testing in patient blood showed the molecule prevented monomer dissociation and reduced toxicity of aggregates. SPARK support helped the team to perform oral dosing studies in animals and show superiority of the small molecule lead to other compounds in development in development for TTR amyloidosis.
With funding from BridgeBio, Dr. Graef founded Eidos Therapeutics, now a public company.
REPURPOSED AND NOVEL SMALL MOLECULES TO IMPROVE MEMORY IN ALZHEIMER’S DISEASE
 Mehrdad Shamloo, MS, PhD
Mehrdad Shamloo, MS, PhD
Associate Professor (research) Of Neurosurgery And, By Courtesy, Of Comparative Medicine And Of Neurology
Dr. Shamloo found that activation of the beta-adrenergic pathway improved cognitive function in animal models of Alzheimer’s disease. Because chronic administration of existing drugs in this pathway can cause heart failure, SPARK advised that Dr. Shamloo in develop novel small molecule activators that are preferentially concentrated in the brain, thereby improving efficacy and minimizing cardiovascular side effects. With funding and advisor support from SPARK, Dr. Shamloo was successful in identifying a development candidate with the desired characteristics. SPARK also supported research to provide further mechanistic understanding pathway, which expanded use of the drug to additional neurodegenerative conditions such as Parkinson’s disease.
Dr. Shamloo founded a start-up company, CuraSen, to advance the drug into the clinic. The company recently closed a $54.5M Series A financing.
REPURPOSED SMALL MOLECULE TO TREAT NOVEL HEPATITIS C TARGET

Jeffrey Glenn, MD, PhD
Associate Professor Of Medicine (Gastroenterology And Hepatology) And Of Microbiology And Immunology
Chronic hepatitis C is a viral infection that can lead to swelling of the liver, non-alcoholic cirrhosis, and liver cancer. Many years before Gilead turned the national conversation around HCV to drug pricing, Dr. Glenn’s team was searching for new ways to inhibit Hepatitis C Virus in infected individuals. They identified a compound, previously tested in humans to treat allergies, that acted synergistically with other HCV protease-inhibitors to block viral replication and slow emergence of drug-resistant viral strains. SPARK funded preclinical studies into the efficacy of the drug combination.
Eiger BioPharmaceuticals, founded by Jeffrey Glenn, MD, PhD, licensed the technology and ran a Phase IB clinical trial to evaluate safety and tolerability in HCV-infected subjects (CLEAN-1 trial, NCT00945880). Although the original product was eclipsed by the Gilead launch of sofosbuvir, Eiger is developing a number of other SPARK and Stanford discoveries. The company, now public (NASDAQ: EIGR), is performing multiple phase II studies around the world.
