
The 2nd edition of SPARK’s trusted and comprehensive book on academic drug development is now available! "A Practical Guide to Drug Development in Academia: The SPARK Approach" is written by SPARK's industry advisors and edited by SPARK co-directors Daria Mochly-Rosen and Kevin Grimes. Updated with the latest developments since the 2014 1st edition, this book is a must-have for academic translational scientists. The electronic book is available now and a hard copy version will be released later this month.
Read more and order the eBookFounder and Director of Stanford University’s SPARK program Daria Mochly-Rosen shares how she has liberated potential drug discoveries from academic research to industry, and discusses the countless, surprising lessons she has learned along the way.
For manuscript submissions, use this sentence in the acknowledgement:
“Mentored and financially supported by Stanford’s SPARK Translational Research Program”
Paper details the SPARK program's goals, methods, and metrics

The 2nd edition of SPARK's authoritative book is now available
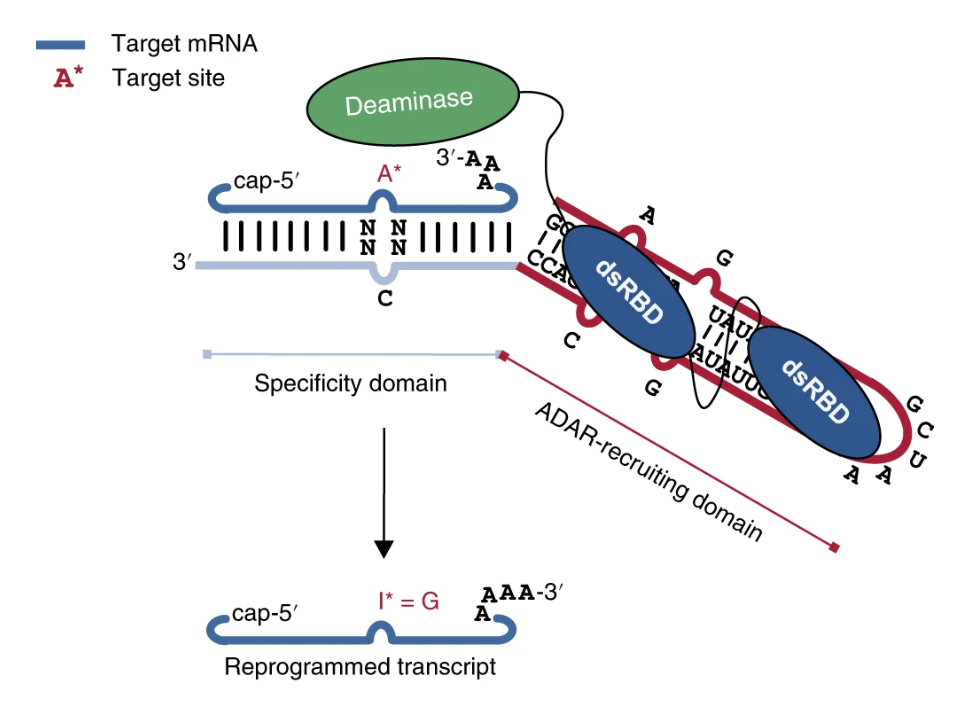
Startup incubated out of SPARK launches to advance RNA editing technology

The SPARK manual was published by co-founders Daria Mochly-Rosen and Kevin Grimes to help other academic institutions develop their own SPARK Programs.
Learn More
SPARK is thankful for the continued support of the Maternal and Child Health Research Institute at Stanford. Through their funding, SPARK has been able fund numerous projects in the field of Child and Maternal Health with 11 projects reaching clinic or commercial sectors.
Learn More
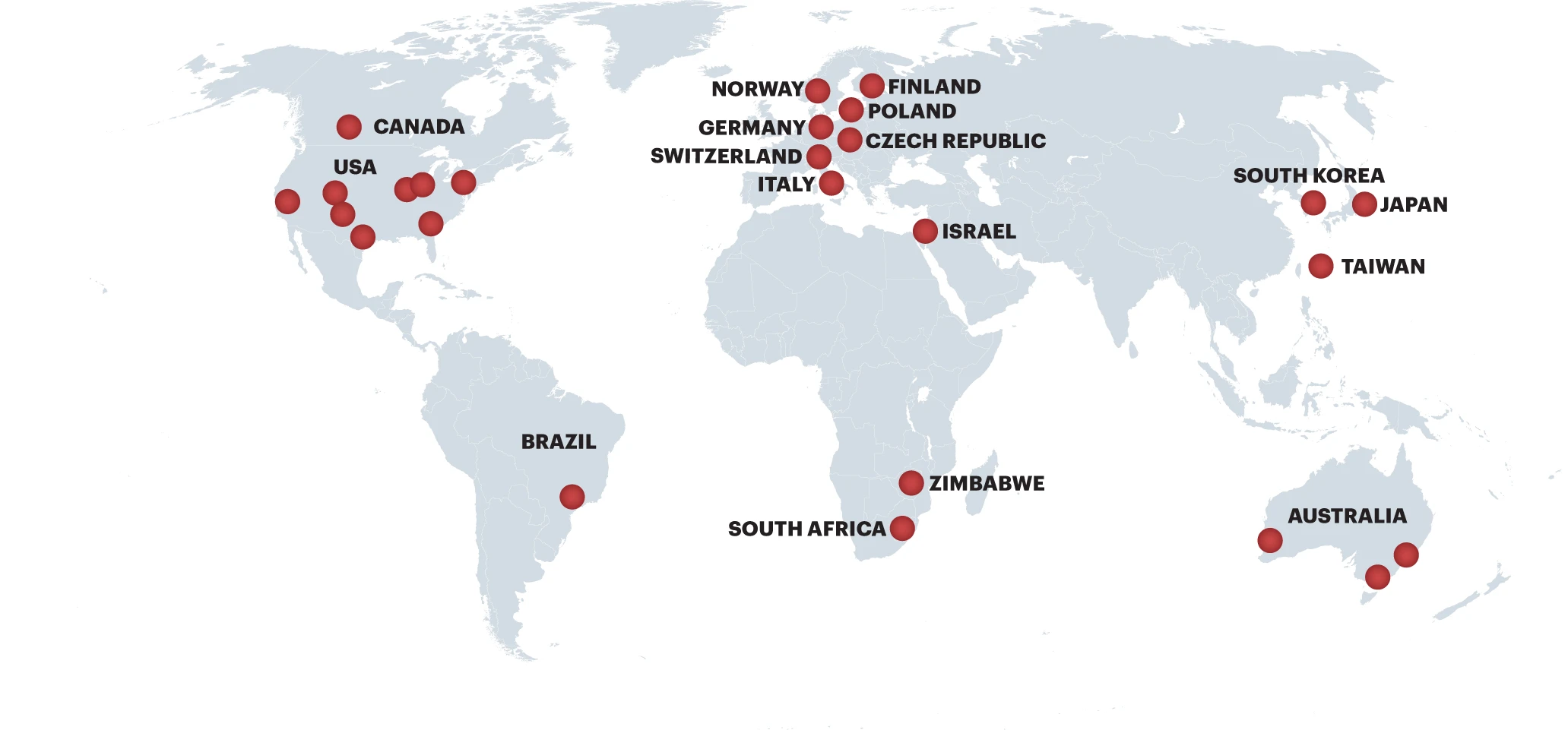
A number of academic institutions have successfully developed their own SPARK programs resulting in a global community of translational scientists who accelerate academic discoveries to patients around the world. Learn more about the work of SPARK Global.
Learn MoreMay15